Are we on the verge of a point-of-care revolution?
21 Apr 2017
Written by Daniel Berman
Diagnostic developers and manufacturers met in Lisbon last week, at this year’s Molecular Diagnostics Europe meeting. The annual event, organised by Cambridge Healthtech Institute (USA), is a place where diagnostic test developers can learn about trends, new technologies and products, while making contacts with potential collaborators.
A recurring theme this year was that the diagnostics industry is on the cusp of a major transformation that will see an increased role for point-of-care (POC) tests that can be run at the bedside, in doctors’ offices, at pharmacies or in people’s homes.
Just as the computing revolution rendered many types of mainframe computers obsolete, as the price of manufacturing POC tests drops and the potential for running multiplex tests in miniature expands, the industry will be transformed.
Point-of-care innovation
In hospitals, many of the new tests will be designed to be run by medical and paramedical professionals rather than lab technicians. This decentralisation of testing will also happen in primary care, driven by patients’ desire for on-the-spot results.
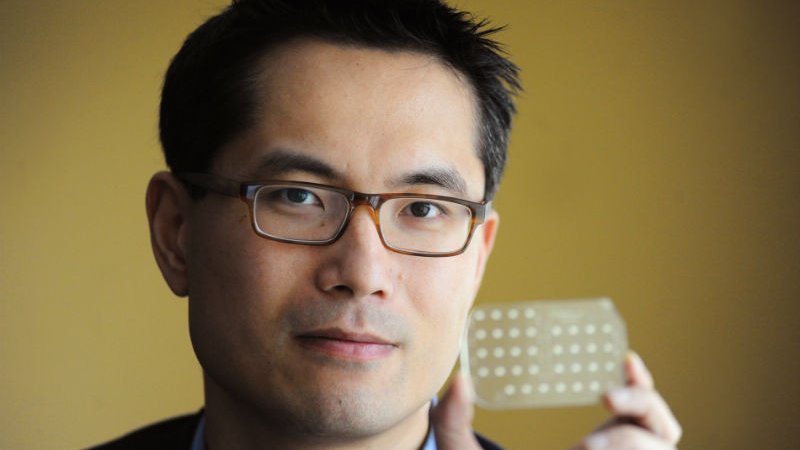
During the POC track of the meeting, Prof Samuel Sia from Columbia University’s Biomedical Engineering School (pictured, above) hit a nerve when he said: “among test developers there are some anxieties because there are so many new tests, yet so few have gotten to market.”
However, he remains optimistic, adding that several factors are converging to increase the chances of success, not least the potential profitability of diagnostics in the advent of personalised medicine.
The diagnostics industry is on the cusp of a major transformation that will see an increased role for point-of-care tests that can be run at the bedside, in doctors’ offices, at pharmacies or in people’s homes.
Not disturbed by the prospect of transformation, Dr Gyorgy Abel, Lab director at Lahey Hospital (USA), said: “We hope we will have some very disruptive technologies.” Stressing the importance of rapid diagnostics in the fight against antibiotic resistance, he added: “The threat of AMR is very real; today totally resistant bacteria are an everyday experience.”
In hospitals, where most tests are run in centralised labs, doctor-decision-makers are frustrated at the long turn-around-time (TAT) of current diagnostics. For some tests, when time to treatment is critical, the demand for point-of-care is growing.
On the other hand, increased use of POCs could help take pressure off hospitals by moving diagnosis to primary care and avoiding unnecessary visits to emergency rooms.
Dr Abel identified influenza and severe respiratory tract infections as priority areas for new POC tests, based on his experience of leading a pathology lab in a US hospital. He also warned against too much emphasis on molecular tests as the Holy Grail, since mutations are evolving and a given test can only look at those genetic strains that have already been identified. As a guideline for new test developers, he said he thought the instrumentation for POCs should cost around $100, with a unit test cost of under $5.00.
Diagnostics outside of the lab
On the primary care side, the tendency to move testing from labs to doctor’s offices or clinics was illustrated by Prof Sia, who has developed an oncology test that’s currently being marketed by OPKO diagnostics. In this case, a urologist does a finger prick and gets a reading on a device within 15 minutes to diagnose prostate cancer.
Sia said: “We wanted to design the test to be used globally but our venture capital investors wanted us to focus on wealthy markets to increase return.” Sia’s solution was to develop a lower cost reader for lower resource markets.
He gave a second example of a test designed for Rwanda. The idea was to bring an ‘Elisa’ test (which measures antibodies in the blood) to a POC ‘microfluidic’ card platform, which can read tiny fluid samples. This test, for HIV and Syphilis, is contained in a dongle that can be plugged into a smartphone jack or communicate via bluetooth. The sample is generated with a finger prick, which then flows through capillaries in the card that hold reagents. Results are available in 15-20 minutes; the cost of the reusable ‘hardware’ is $34; the test uses 100,000x less power than a classic elisa and has 80-90% sensitivity and specificity. Prof Sia argued that this was adequate, since the test would dramatically increase access.
Liesbet Lagae, PhD. from IMEC (Belgium) explained that many of the tests performed today in labs could move to POC versions that rely on sensors, which could be produced in miniature formats to drive down costs. This technology is called lab-on-chip (LOC), and can purify and analyse a sample (blood, urine, saliva) just like a clinical lab does. IMEC’s focus is on LOCs made partly or entirely from silicon, the same material computer chips are made from, which can significantly reduce manufacturing costs.
Point-of-care tests to tackle AMR
The POC track in Lisbon also focused directly on AMR. Till Bachmann, Ph.D, Reader at University of Edinburgh, shared that according to the UK Review on Antimicrobial Resistance, if current trends continue, by 2050 there will be 10 million deaths attributed to the failure of antibiotics. Development of new diagnostics was cited among the 10 priorities for tackling AMR, and by 2020 the report advocated that no prescription should be written for antibiotics without diagnostic testing.
Bachmann also urged for a change of paradigm that would end today’s two-step practice for diagnostic testing: 1) identify bacteria; and 2) explore resistance levels to potential treatments. He encouraged developers to meet the challenge of developing tests that would fuse the two steps and produce results in real time.
Throughout the event, it was clear that the field of POC diagnostics is exploding with new ideas, techniques and innovative funding. But it’s still too early to see which new technologies or ideas will dominate in hospitals and clinics in the years to come.
Image credit: Colombia University Biomedical Engineering School
