Sex, Drugs and Superbugs: Gonorrhoea and the post-antibiotic apocalypse
13 Aug 2018
Written by Dr. Tina Joshi, University of Plymouth
The UK declared its first ever case of “super” resistant gonorrhoea this past March (2018). The patient in question had contracted the bacterium after an unprotected sexual encounter in South East Asia and upon his return, the infection failed to respond to the first choice antibiotic treatments – azithromycin and ceftriaxone. This was also Public Health England’s first global report of the infection resisting both of these antibiotics and it has now, justifiably, caused serious concern for clinicians and scientists on a global scale.
What is super gonorrhea and why is it spreading?
Gonorrhoea is a sexually transmitted infection (STI) caused by the bacterium Neisseria gonorrhoea that can be symptomless, but can lead to infertility in both men and women if left untreated. Those with symptoms show unusual discharge from sexual organs and inflammation within them. The infection can be transmitted via oral, vaginal and anal sex, and even during pregnancy to an unborn foetus.
Multi-drug resistant gonorrhoea is easier to pick up in countries where antibiotics can be easily purchased over the counter and used freely without the advice of a doctor or a way to know if it is the right antibiotic for an infection or if the infection is, in fact, bacterial. This means that antibiotics are used inappropriately or overused, which increases overall rates of resistance. Recently, Australia reported two patients who were infected with “super” gonorrhoea and are also believed to have contracted the bug in South East Asia, suggesting that these superbugs may be circulating in this part of the world and thus may become more common in future.
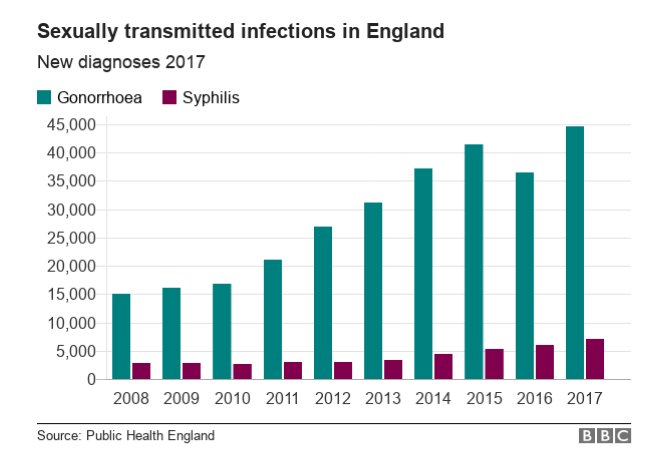
In 2017, approximately 7,000 cases of syphilis, 46,000 cases of gonorrhoea and 200,000 cases of chlamydia were reported to Public Health England (PHE). According to the Office of National Statistics, this is an increase in cases of gonorrhoea and syphilis in England compared with 2016.
Chlamydia is the most commonly diagnosed sexually transmitted infection (STI) in England and is treatable with antibiotics for the moment – but is still capable of causing lasting fertility issues. While cases of syphilis are uncommon, an increase in cases is still a cause for concern considering syphilis was eradicated after WWII. We also cannot forget the warnings about antibiotic resistance developing in the little-known new STI Mycoplasma genitalium or MGen which were made by the British Association of Sexual Health and HIV. MGen is rarely diagnosed and tested for, but equally has severe consequences of causing infertility in women.
Apparently 44% of those surveyed didn’t feel the need to get tested because they are symptomless, and often in the heat of the moment many people don’t use protection.
Changes in sexual behaviour have been linked to this rise in infections due to multiple factors; one of which is the advent of online dating and an increased acceptance around casual sex. This is not limited to 16-24 year olds, as there is also a rise in STIs in the over 50s too. Another factor has been attributed to more people attending STI clinics and using DIY home test kits.
Perhaps the most important way to stop transmission of these infections is to practise safe sex – and this also means the practise of safe oral sex as these superbugs can also infect the throat. But when one in five 18-24 years olds in the UK do not know what an STI is (according to a survey of 1,000 people in this age group by charity The Mix) how do we tackle the problem?
Are we educating appropriately? Apparently 44% of those surveyed didn’t feel the need to get tested because they are symptomless, and often in the heat of the moment most people don’t use protection. With the news that there are budget cuts to NHS STI clinics and clinical testing, it makes one wonder just how many symptomless patients are flying under the radar.
When the drugs don’t work
Antibiotic resistant “superbugs” have been talked about on a public platform for some years now, however there is still confusion and misconceptions about the severity of the antibiotic crisis. The patient above with “super” gonorrhoea was successfully treated with one of the last line antibiotics remaining in our arsenal; ertapenem. Does this mean we do not need to worry about infections because we surely must have some working antibiotics left?
No.
What is worrying is that we really do not. And the “we” here is the human species. It was diligence (and luck) on the behalf of the healthcare professionals who found the right antibiotic to treat that patient.
Infectious bacteria across the spectrum are becoming antibiotic resistant. One of the biggest misconceptions is that people themselves are becoming resistant to antibiotics. This is not true. The bacteria are becoming resistant to the antibiotics. Bacteria, like E. coli, reproduce more bacteria every 20 minutes compared to humans who tend to reproduce every 30 years on average. This means that E. coli will generate the same amount of bacteria in 2.5 years as we could generate in 2 million years. Taking this into account, it’s hardly surprising that bacteria evolve to survive antibiotic treatment, and that their DNA will be able to acquire resistance genes (genetic exchange).
Antibiotics are usually given for severe infection; however inappropriate antibiotic prescribing has contributed to resistance. In some cases when broad-spectrum antibiotics are used at inappropriate doses, most of the bacteria are killed but not all. This means that the surviving bacteria become resistant and pass on this resistance to their offspring.
Scientists and organisations around the globe are trying to combat the rise of antibiotic resistance. However, to make progress there needs to be investment in research to find new antibiotics, develop faster diagnostics and educate healthcare professionals.
The WHO has warned that we as a species are now facing the prospect of returning to a “pre-antibiotic” era similar to the “dark ages”. This is scientific fact, not fiction, and it’s important for the public to understand the truth about antibiotics. Antibiotics are used in many medical processes including cancer biopsies, giving birth, heart bypasses and hip replacements, just to name just a few. When a person has an infection and that infection becomes antibiotic resistant and untreatable then there is a real possibility of death. Infection could come from just a simple scratch…
The O’Neil AMR Report of 2016 estimates that by 2050, 10 million people per year will die as a direct result of antibiotic resistant infections. This is only just starting to affect our generation and will certainly affect future generations if humans don’t find ways of solving the problem.
Scientists and organisations around the globe are trying to combat the rise of antibiotic resistance. However, to make progress there needs to be investment in research to find new antibiotics, develop faster diagnostics and educate healthcare professionals. Perhaps the best strategy is to preserve the antibiotics that we have left by sparing their use. A great analogy was recently used by Hall, McDonnell and O’Neil in their book “Superbugs: An arms race against bacteria”stating that antibiotics should be conserved as a non-renewable natural resource in a similar vein to fossil fuels. “Why?” you may ask.
Antibiotics should be treated like non-renewable resources
Antibiotic discovery and subsequent drug development is not that simple; it takes 10-20 years to bring new drugs to market, costing in excess of $1 billion in clinical trials and testing. A company might have a monopoly over the drug due to intellectual property rights, with investment costs being recouped after 10 years. Beyond this the drug can be sold by other manufacturers at a reduced price and profits will be made. This is a long process and there is no guarantee that a company will take on a newly discovered antibiotic due to the high investment. This is especially so considering in the past months several well-known drug companies have actually shut their antibiotic and antiviral research and development projects.
Currently, Merck, Roche, GlaxoSmithKline and Pfizer are the large remaining pharmaceutical companies with active antibiotic research programs. However, it is usually the small startup companies that develop antibiotics and these startups are acquired by big pharma who then take the product to market. But if a lot of big pharma companies are not invested in tackling the antibiotic crisis, this is leaves us with less variety of potential antibiotics that can be taken to market.
If an antibiotic drug was invested in, it would likely be protected by public health officials for use in extreme circumstances to prevent any resistance from occurring; the resource would be precious and according to Dame Sally Davies (Chief Medical Officer for England) appropriate “stewardship programs” would need to be implemented. Indeed, the pipeline of new antibiotics is not as strong as it should be and this is largely due to the economics described above. For example, the World Health Organisation (WHO) expressed concern that only three antibiotics are in development for “super” gonorrhoea.
Dr. Tina Joshi is Lecturer in Molecular Microbiology at the University of Plymouth teaching clinical microbiology and antibiotic stewardship at the Faculty of Medicine & Dentistry. Tina specialises in development of novel rapid point of care detection systems for antibiotic resistant bacteria and in infection control. Recently Tina was awarded the Hind Rattan “Jewel of India” award for her research and has been elected to the Microbiology Society Policy Committee in an effort to influence government policy. Her research is soon to be featured in a BBC documentary due to be aired in Autumn 2018. Email: Tina.Joshi@plymouth.ac.uk Twitter: @tinaljoshi
